Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility
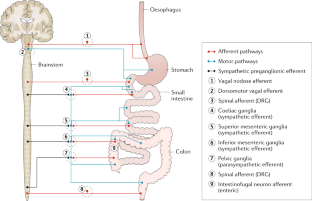
The gastrointestinal tract is the only internal organ to have evolved with its own independent nervous system, known as the enteric nervous system (ENS). This Review provides an update on advances that have been made in our understanding of how neurons within the ENS coordinate sensory and motor functions. Understanding this function is critical for determining how deficits in neurogenic motor patterns arise. Knowledge of how distension or chemical stimulation of the bowel evokes sensory responses in the ENS and central nervous system have progressed, including critical elements that underlie the mechanotransduction of distension-evoked colonic peristalsis. Contrary to original thought, evidence suggests that mucosal serotonin is not required for peristalsis or colonic migrating motor complexes, although it can modulate their characteristics. Chemosensory stimuli applied to the lumen can release substances from enteroendocrine cells, which could subsequently modulate ENS activity. Advances have been made in optogenetic technologies, such that specific neurochemical classes of enteric neurons can be stimulated. A major focus of this Review will be the latest advances in our understanding of how intrinsic sensory neurons in the ENS detect and respond to sensory stimuli and how these mechanisms differ from extrinsic sensory nerve endings in the gut that underlie the gut–brain axis.
Key points
- In vertebrates, the enteric nervous system (ENS) is critical for gastrointestinal function.
- There has been much progress in understanding the mechanisms by which mechanical or chemical stimulation of the gut is converted into neural activity within the ENS and propulsive motility.
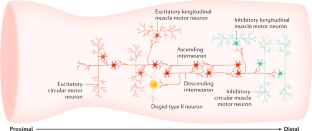
- Mechanosensory elements critical for distension-evoked colonic peristalsis have been identified to lie in the myenteric plexus and/or circular muscle of the gastrointestinal tract and do not require the mucosa or submucosal plexus.
- Evidence suggests that substances released from cells in the mucosa (such as enteroendocrine cells) can modulate ENS activity, but the release of mediators like serotonin is not required for distension-evoked peristalsis or for colonic migrating motor complexes.
- Fundamental differences have been revealed in the mechanisms of activation of extrinsic spinal afferent nerves compared with those of intrinsic sensory nerves in the same region of the bowel.
- Recent refinements in optogenetic technologies now permit the stimulation of specific neurochemical classes of neurons in the ENS to elucidate function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
206,07 € per year
only 17,17 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Neural signalling of gut mechanosensation in ingestive and digestive processes
Article 04 January 2022
Gut feelings: mechanosensing in the gastrointestinal tract
Article 12 January 2022

Microbiota modulate sympathetic neurons via a gut–brain circuit
Article 08 July 2020
References
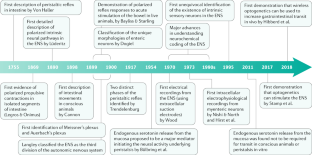
- Von Haller, A. A Dissertation on the Sensible and Irritable Parts of Animals (1755), republished in Bulletin of the Institute of the History of Medicine4, 651–699 (1936).
- Kunze, W. A., Bornstein, J. C. & Furness, J. B. Identification of sensory nerve cells in a peripheral organ (the intestine) of a mammal. Neuroscience66, 1–4 (1995). CASPubMedGoogle Scholar
- Furness, J. B., Johnson, P. J., Pompolo, S. & Bornstein, J. C. Evidence that enteric motility reflexes can be initiated through entirely intrinsic mechanisms in the guinea-pig small intestine. Neurogastroenterol. Motil.7, 89–96 (1995). CASPubMedGoogle Scholar
- Trendelenburg, P. Physiologische und pharmakologische Untersuchungen über die Dünndarmperistaltik. Arch. Exp. Pathol. Pharmakol.81, 55–129 (1917). Google Scholar
- Wood, J. D. Electrical activity of the intestine of mice with hereditary megacolon and absence of enteric ganglion cells. Am. J. Dig. Dis.18, 477–488 (1973). CASPubMedGoogle Scholar
- Costa, M. & Furness, J. B. The peristaltic reflex: an analysis of the nerve pathways and their pharmacology. Naunyn Schmiedeberg’s Arch. Pharmacol.294, 47–60 (1976). CASGoogle Scholar
- Lüderitz, C. Experimentelle untersuchungen uber die Entstehung der darmperistaltik. Arch. Path. Anat. Physiol. Klin. Med.122, 1–28 (1890). Google Scholar
- Lüderitz, C. Das motorische Verhalten des Magens bei Reizung seiner ausseren Flache. Arch. Ges. Physiol. Men. Tiere49, 158–174 (1891). Google Scholar
- Bayliss, W. M. & Starling, E. H. The movements and innervation of the small intestine. J. Physiol.24, 99–143 (1899). CASPubMedPubMed CentralGoogle Scholar
- Bartho, L., Holzer, P., Donnerer, J. & Lembeck, F. Evidence for the involvement of substance P in the atropine-resistant peristalsis of the guinea-pig ileum. Neurosci. Lett.32, 69–74 (1982). CASPubMedGoogle Scholar
- Smith, T. K. & Robertson, W. J. Synchronous movements of the longitudinal and circular muscle during peristalsis in the isolated guinea-pig distal colon. J. Physiol.506, 563–577 (1998). CASPubMedPubMed CentralGoogle Scholar
- Spencer, N. J. & Smith, T. K. Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig distal colon. J. Physiol.533, 787–799 (2001). CASPubMedPubMed CentralGoogle Scholar
- Tonini, M. et al. 5-HT7 receptors modulate peristalsis and accommodation in the guinea pig ileum. Gastroenterology129, 1557–1566 (2005). CASPubMedGoogle Scholar
- Furness, J. B. The Enteric Nervous System (Blackwell, 2006).
- Balasuriya, G. K., Hill-Yardin, E. L., Gershon, M. D. & Bornstein, J. C. A sexually dimorphic effect of cholera toxin: rapid changes in colonic motility mediated via a 5-HT3 receptor-dependent pathway in female C57Bl/6 mice. J. Physiol.594, 4325–4338 (2016). CASPubMedPubMed CentralGoogle Scholar
- Spencer, N. J., Dinning, P. G., Brookes, S. J. & Costa, M. Insights into the mechanisms underlying colonic motor patterns. J. Physiol.594, 4099–4116 (2016). CASPubMedPubMed CentralGoogle Scholar
- Hu, H. & Spencer, N. J. in Physiology of the Gastrointestinal Tract 6th edn Vol. 1 Ch. 14 (ed. Said, H. M.) 629–669 (Elsevier/Academic Press, 2018).
- Kuizenga, M. H. et al. Neurally mediated propagating discrete clustered contractions superimposed on myogenic ripples in ex vivo segments of human ileum. Am. J. Physiol. Gastrointest. Liver Physiol.308, G1–G11 (2015). CASPubMedGoogle Scholar
- Spencer, N. J. et al. Characterization of motor patterns in isolated human colon: are there differences in patients with slow-transit constipation? Am. J. Physiol. Gastrointest. Liver Physiol.302, G34–G43 (2012). CASPubMedGoogle Scholar
- Weakly, J. N. Similarites in synaptic efficacy along multiply innervated twich muscle fibers of the frog: a possible muscle-to-motoneuron interaction. Brain Res.158, 235–239 (1978). CASPubMedGoogle Scholar
- Bennett, M. R., Burnstock, G. & Holman, M. E. Transmission from perivascular inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. J. Physiol.182, 527–540 (1966). CASPubMedPubMed CentralGoogle Scholar
- Bulbring, E. & Tomita, T. Properties of the inhibitory potential of smooth muscle as observed in the response to field stimulation of the guinea-pig taenia coli. J. Physiol.189, 299–315 (1967). CASPubMedPubMed CentralGoogle Scholar
- Furness, J. B. Types of neurons in the enteric nervous system. J. Auton. Nerv. Syst.81, 87–96 (2000). CASPubMedGoogle Scholar
- Brookes, S. J. Classes of enteric nerve cells in the guinea-pig small intestine. Anat. Rec.262, 58–70 (2001). CASPubMedGoogle Scholar
- Costa, M., Furness, J. B. & Gibbins, I. L. Chemical coding of enteric neurons. Prog. Brain Res.68, 217–239 (1986). CASPubMedGoogle Scholar
- Costa, M. et al. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience75, 949–967 (1996). CASPubMedGoogle Scholar
- Brookes, S. J., Song, Z. M., Ramsay, G. A. & Costa, M. Long aboral projections of Dogiel type II, AH neurons within the myenteric plexus of the guinea pig small intestine. J. Neurosci.15, 4013–4022 (1995). CASPubMedPubMed CentralGoogle Scholar
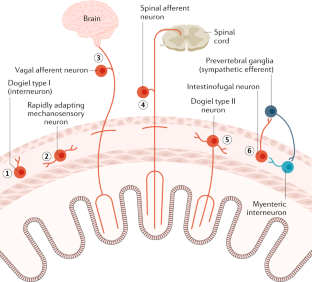
- Furness, J. B., Kunze, W. A., Bertrand, P. P., Clerc, N. & Bornstein, J. C. Intrinsic primary afferent neurons of the intestine. Prog. Neurobiol.54, 1–18 (1998). CASPubMedGoogle Scholar
- Ro, S., Hwang, S. J., Muto, M., Jewett, W. K. & Spencer, N. J. Anatomic modifications in the enteric nervous system of piebald mice and physiological consequences to colonic motor activity. Am. J. Physiol. Gastrointest. Liver Physiol.290, G710–G718 (2006). CASPubMedGoogle Scholar
- Burnett, A. L. & Diehl, N. A. The nervous system of Hydra. I. Types, distribution and origin of nerve elements. J. Exp. Zool.157, 217–226 (1964). CASPubMedGoogle Scholar
- Murillo-Rincon, A. P. et al. Spontaneous body contractions are modulated by the microbiome of Hydra. Sci. Rep.7, 15937 (2017). PubMedPubMed CentralGoogle Scholar
- Obermayr, F., Hotta, R., Enomoto, H. & Young, H. M. Development and developmental disorders of the enteric nervous system. Nat. Rev. Gastroenterol. Hepatol.10, 43–57 (2013). CASPubMedGoogle Scholar
- Young, H. M. & McKeown, S. J. Motility: Hirschsprung disease–laying down a suitable path. Nat. Rev. Gastroenterol. Hepatol.13, 7–8 (2016). CASPubMedGoogle Scholar
- Stamp, L. A. et al. Optogenetic demonstration of functional innervation of mouse colon by neurons derived from transplanted neural cells. Gastroenterology152, 1407–1418 (2017). This paper was the first to demonstrate that light could be used to excite enteric neurons using optogenetics. PubMedGoogle Scholar
- Hotta, R. et al. Transplanted progenitors generate functional enteric neurons in the postnatal colon. J. Clin. Invest.123, 1182–1191 (2013). CASPubMedPubMed CentralGoogle Scholar
- Hetz, S. et al. In vivo transplantation of neurosphere-like bodies derived from the human postnatal and adult enteric nervous system: a pilot study. PLoS One9, e93605 (2014). PubMedPubMed CentralGoogle Scholar
- Cooper, J. E. et al. In vivo transplantation of enteric neural crest cells into mouse gut; engraftment, functional integration and long-term safety. PLoS One11, e0147989 (2016). PubMedPubMed CentralGoogle Scholar
- Metzger, M., Caldwell, C., Barlow, A. J., Burns, A. J. & Thapar, N. Enteric nervous system stem cells derived from human gut mucosa for the treatment of aganglionic gut disorders. Gastroenterology136, 2214–2225 (2009). CASPubMedGoogle Scholar
- Nishikawa, R. et al. Migration and differentiation of transplanted enteric neural crest-derived cells in murine model of Hirschsprung’s disease. Cytotechnology67, 661–670 (2015). CASPubMedGoogle Scholar
- Fattahi, F. et al. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature531, 105–109 (2016). CASPubMedPubMed CentralGoogle Scholar
- McCann, C. J. et al. Transplantation of enteric nervous system stem cells rescues nitric oxide synthase deficient mouse colon. Nat. Commun.8, 15937 (2017). This exciting in vivo study demonstrated that enteric neural stem cells can be successfully transplanted and integrated into the ENS to restore nitrergic neurons and function in mutant mice genetically deficient in neuronal nitric oxide. CASPubMedPubMed CentralGoogle Scholar
- Pham, T. D., Gershon, M. D. & Rothman, T. P. Time of origin of neurons in the murine enteric nervous system: sequence in relation to phenotype. J. Comp. Neurol.314, 789–798 (1991). CASPubMedGoogle Scholar
- Kulkarni, S. et al. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc. Natl Acad. Sci. USA114, E3709–E3718 (2017). CASPubMedGoogle Scholar
- Corpening, J. C., Cantrell, V. A., Deal, K. K. & Southard-Smith, E. M. A Histone2BCerulean BAC transgene identifies differential expression of Phox2b in migrating enteric neural crest derivatives and enteric glia. Dev. Dyn.237, 1119–1132 (2008). CASPubMedPubMed CentralGoogle Scholar
- Gianino, S., Grider, J. R., Cresswell, J., Enomoto, H. & Heuckeroth, R. O. GDNF availability determines enteric neuron number by controlling precursor proliferation. Development130, 2187–2198 (2003). CASPubMedGoogle Scholar
- Furness, J. B., Kuramoto, H. & Messenger, J. P. Morphological and chemical identification of neurons that project from the colon to the inferior mesenteric ganglia in the guinea-pig. J. Auton. Nerv. Syst.31, 203–210 (1990). CASPubMedGoogle Scholar
- Rao, M. & Gershon, M. D. Neurogastroenterology: the dynamic cycle of life in the enteric nervous system. Nat. Rev. Gastroenterol. Hepatol.14, 453–454 (2017). PubMedGoogle Scholar
- Dickens, E. J., Hirst, G. D. & Tomita, T. Identification of rhythmically active cells in guinea-pig stomach. J. Physiol.514, 515–531 (1999). CASPubMedPubMed CentralGoogle Scholar
- Der-Silaphet, T., Malysz, J., Hagel, S., Larry Arsenault, A. & Huizinga, J. D. Interstitial cells of cajal direct normal propulsive contractile activity in the mouse small intestine. Gastroenterology114, 724–736 (1998). CASPubMedGoogle Scholar
- Ward, S. M., Burns, A. J., Torihashi, S. & Sanders, K. M. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J. Physiol.480, 91–97 (1994). CASPubMedPubMed CentralGoogle Scholar
- Huizinga, J. D. et al. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature373, 347–349 (1995). CASPubMedGoogle Scholar
- Ward, S. M. et al. Development of interstitial cells of Cajal and pacemaking in mice lacking enteric nerves. Gastroenterology117, 584–594 (1999). CASPubMedGoogle Scholar
- Gershon, M. D. Lessons from genetically engineered animal models. II. Disorders of enteric neuronal development: insights from transgenic mice. Am. J. Physiol.277, G262–G267 (1999). CASPubMedGoogle Scholar
- Spencer, N. J., Sanders, K. M. & Smith, T. K. Migrating motor complexes do not require electrical slow waves in the mouse small intestine. J. Physiol.553, 881–893 (2003). CASPubMedPubMed CentralGoogle Scholar
- Ward, S. M. et al. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J. Neurosci.20, 1393–1403 (2000). CASPubMedPubMed CentralGoogle Scholar
- Klein, S. et al. Interstitial cells of Cajal integrate excitatory and inhibitory neurotransmission with intestinal slow-wave activity. Nat. Commun.4, 1630 (2013). PubMedGoogle Scholar
- Goyal, R. K. & Chaudhury, A. Mounting evidence against the role of ICC in neurotransmission to smooth muscle in the gut. Am. J. Physiol. Gastrointest. Liver Physiol.298, G10–G13 (2010). CASPubMedGoogle Scholar
- Goyal, R. K. CrossTalk opposing view: interstitial cells are not involved and physiologically important in neuromuscular transmission in the gut. J. Physiol.594, 1511–1513 (2016). CASPubMedPubMed CentralGoogle Scholar
- Goyal, R. K. Rebuttal from Raj K Goyal. J. Physiol.594, 1517 (2016). PubMedPubMed CentralGoogle Scholar
- Zhang, R. X., Wang, X. Y., Chen, D. & Huizinga, J. D. Role of interstitial cells of Cajal in the generation and modulation of motor activity induced by cholinergic neurotransmission in the stomach. Neurogastroenterol. Motil.23, e356–e371 (2011). PubMedGoogle Scholar
- Zhang, Y., Carmichael, S. A., Wang, X. Y., Huizinga, J. D. & Paterson, W. G. Neurotransmission in lower esophageal sphincter of W/Wv mutant mice. Am. J. Physiol. Gastrointest. Liver Physiol.298, G14–G24 (2010). CASPubMedGoogle Scholar
- Driessen, A. K., Farrell, M. J., Mazzone, S. B. & McGovern, A. E. Multiple neural circuits mediating airway sensations: Recent advances in the neurobiology of the urge-to-cough. Respir. Physiol. Neurobiol.226, 115–120 (2016). PubMedGoogle Scholar
- Nishi, S. & North, R. A. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J. Physiol.231, 471–491 (1973). CASPubMedPubMed CentralGoogle Scholar
- Hirst, G. D., Holman, M. E. & Spence, I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J. Physiol.236, 303–326 (1974). CASPubMedPubMed CentralGoogle Scholar
- Kunze, W. A., Furness, J. B., Bertrand, P. P. & Bornstein, J. C. Intracellular recording from myenteric neurons of the guinea-pig ileum that respond to stretch. J. Physiol.506, 827–842 (1998). CASPubMedPubMed CentralGoogle Scholar
- Bornstein, J. C., Furness, J. B. & Kunze, W. A. Electrophysiological characterization of myenteric neurons: how do classification schemes relate? J. Auton. Nerv. Syst.48, 1–15 (1994). CASPubMedGoogle Scholar
- Furness, J. B., Robbins, H. L., Xiao, J., Stebbing, M. J. & Nurgali, K. Projections and chemistry of Dogiel type II neurons in the mouse colon. Cell Tissue Res.317, 1–12 (2004). CASPubMedGoogle Scholar
- Bornstein, J. C., Hendriks, R., Furness, J. B. & Trussell, D. C. Ramifications of the axons of AH-neurons injected with the intracellular marker biocytin in the myenteric plexus of the guinea pig small intestine. J. Comp. Neurol.314, 437–451 (1991). CASPubMedGoogle Scholar
- Mao, Y., Wang, B. & Kunze, W. Characterization of myenteric sensory neurons in the mouse small intestine. J. Neurophysiol.96, 998–1010 (2006). PubMedGoogle Scholar
- Spencer, N. J. & Smith, T. K. Mechanosensory S-neurons rather than AH-neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. J. Physiol.558, 577–596 (2004). CASPubMedPubMed CentralGoogle Scholar
- Smith, T. K., Spencer, N. J., Hennig, G. W. & Dickson, E. J. Recent advances in enteric neurobiology: mechanosensitive interneurons. Neurogastroenterol. Motil.19, 869–878 (2007). CASPubMedGoogle Scholar
- Mazzuoli-Weber, G. & Schemann, M. Mechanosensitivity in the enteric nervous system. Front. Cell. Neurosci.9, 408 (2015). PubMedPubMed CentralGoogle Scholar
- Dogiel, A. S. Über den Bau der Ganglien in den Geflechten des Darmes und der Gallenblase des Menschen und der Säugetiere. Arch. Anat. Physiol. Anat. Abt.1899, 130–158 (1899). Google Scholar
- Mazzuoli, G. & Schemann, M. Mechanosensitive enteric neurons in the myenteric plexus of the mouse intestine. PLoS One7, e39887 (2012). CASPubMedPubMed CentralGoogle Scholar
- Kugler, E. M. et al. Mechanical stress activates neurites and somata of myenteric neurons. Front. Cell. Neurosci.9, 342 (2015). PubMedPubMed CentralGoogle Scholar
- Mazzuoli-Weber, G. & Schemann, M. Mechanosensitive enteric neurons in the guinea pig gastric corpus. Front. Cell. Neurosci.9, 430 (2015). PubMedPubMed CentralGoogle Scholar
- Kugler, E. M. et al. Sensitivity to strain and shear stress of isolated mechanosensitive enteric neurons. Neuroscience372, 213–224 (2018). This study revealed that shear stress was not an adequate stimulus for activation of mechanosensitive enteric neurons; however, strain was sufficient to activate mechanosensitive enteric neurons. CASPubMedGoogle Scholar
- Kunze, W. A., Clerc, N., Bertrand, P. P. & Furness, J. B. Contractile activity in intestinal muscle evokes action potential discharge in guinea-pig myenteric neurons. J. Physiol.517, 547–561 (1999). CASPubMedPubMed CentralGoogle Scholar
- Bertrand, P. P., Kunze, W. A., Bornstein, J. C., Furness, J. B. & Smith, M. L. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am. J. Physiol.273, G422–G435 (1997). CASPubMedGoogle Scholar
- Smolilo, D. J., Costa, M., Hibberd, T. J., Wattchow, D. A. & Spencer, N. J. Morphological evidence for novel enteric neuronal circuitry in guinea pig distal colon. J. Comp. Neurol.526, 1662–1672 (2018). CASPubMedGoogle Scholar
- Crowcroft, P. J., Holman, M. E. & Szurszewski, J. H. Excitatory input from the colon to the inferior mesenteric ganglion. J. Physiol.208, 19P–20P (1970). CASPubMedGoogle Scholar
- Miller, S. M. & Szurszewski, J. Physiology of prevertebral ganglia. Physiol. Gastrointest. Tract.19, 795–877 (1994). Google Scholar
- Bywater, R. A. Activity following colonic distension in enteric sensory fibres projecting to the inferior mesenteric ganglion in the guinea pig. J. Auton. Nerv. Syst.46, 19–26 (1994). CASPubMedGoogle Scholar
- Stebbing, M. J. & Bornstein, J. C. Electrophysiological analysis of the convergence of peripheral inputs onto neurons of the coeliac ganglion in the guinea pig. J. Auton. Nerv. Syst.46, 93–105 (1994). CASPubMedGoogle Scholar
- Miller, S. M. & Szurszewski, J. H. Colonic mechanosensory afferent input to neurons in the mouse superior mesenteric ganglion. Am. J. Physiol.272, G357–G366 (1997). CASPubMedGoogle Scholar
- Jiang, Z., Dun, N. J. & Karczmar, A. G. Substance P: a putative sensory transmitter in mammalian autonomic ganglia. Science217, 739–741 (1982). CASPubMedGoogle Scholar
- Kreulen, D. L. & Szurszewski, J. H. Reflex pathways in the abdominal prevertebral ganglia: evidence for a colo-colonic inhibitory reflex. J. Physiol.295, 21–32 (1979). CASPubMedPubMed CentralGoogle Scholar
- Miller, S. M. & Szurszewski, J. H. Circumferential, not longitudinal, colonic stretch increases synaptic input to mouse prevertebral ganglion neurons. Am. J. Physiol. Gastrointest. Liver Physiol285, G1129–G1138 (2003). CASPubMedGoogle Scholar
- Lynn, P., Zagorodnyuk, V., Hennig, G., Costa, M. & Brookes, S. Mechanical activation of rectal intraganglionic laminar endings in the guinea pig distal gut. J. Physiol.564, 589–601 (2005). CASPubMedPubMed CentralGoogle Scholar
- Hibberd, T. J., Zagorodnyuk, V. P., Spencer, N. J. & Brookes, S. J. Viscerofugal neurons recorded from guinea-pig colonic nerves after organ culture. Neurogastroenterol. Motil.24, 1041-e548 (2012). CASPubMedGoogle Scholar
- Miller, S. M. & Szurszewski, J. H. Relationship between colonic motility and cholinergic mechanosensory afferent synaptic input to mouse superior mesenteric ganglion. Neurogastroenterol. Motil.14, 339–348 (2002). CASPubMedGoogle Scholar
- Lynn, P. A., Olsson, C., Zagorodnyuk, V., Costa, M. & Brookes, S. J. H. Rectal intraganglionic laminar endings are transduction sites of extrinsic mechanoreceptors in the guinea pig rectum. Gastroenterology125, 786–794 (2003). PubMedGoogle Scholar
- Spencer, N. J. et al. Identification of capsaicin-sensitive rectal mechanoreceptors activated by rectal distension in mice. Neuroscience153, 518–534 (2008). CASPubMedPubMed CentralGoogle Scholar
- Zagorodnyuk, V. P., Kyloh, M., Brookes, S. J., Nicholas, S. J. & Spencer, N. J. Firing patterns and functional roles of different classes of spinal afferents in rectal nerves during colonic migrating motor complexes in mouse colon. Am. J. Physiol. Gastrointest. Liver Physiol.303, G404–G411 (2012). CASPubMedGoogle Scholar
- Zagorodnyuk, V. P., Lynn, P., Costa, M. & Brookes, S. J. Mechanisms of mechanotransduction by specialized low-threshold mechanoreceptors in the guinea pig rectum. Am. J. Physiol. Gastrointest. Liver Physiol.289, G397–G406 (2005). CASPubMedGoogle Scholar
- Feng, J. et al. Piezo2 channel-Merkel cell signaling modulates the conversion of touch to itch. Science360, 530–533 (2018). CASPubMedPubMed CentralGoogle Scholar
- Hibberd, T. J., Zagorodnyuk, V. P., Spencer, N. J. & Brookes, S. J. Identification and mechanosensitivity of viscerofugal neurons. Neuroscience225, 118–129 (2012). CASPubMedGoogle Scholar
- Büllbring, E. & Lin, R. C. The action of 5-hydroxytryptamine (5-HT) on peristalsis. J. Physiol.138, 12P (1957). Google Scholar
- Büllbring, E. & Lin, R. C. The effect of intraluminal application of 5-hydroxytryptamine and 5-hydroxytryptophan on peristalsis; the local production of 5-HT and its release in relation to intraluminal pressure and propulsive activity. J. Physiol.140, 381–407 (1958). Google Scholar
- Büllbring, E., Lin, R. C. & Schofield, G. An investigation of the peristaltic reflex in relation to anatomical observations. Q. J. Exp. Physiol. Cogn. Med. Sci.43, 26–43 (1958). Google Scholar
- Jin, J. G., Foxx-Orenstein, A. E. & Grider, J. R. Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. J. Pharmacol. Exp. Ther.288, 93–97 (1999). CASPubMedGoogle Scholar
- Kadowaki, M., Wade, P. R. & Gershon, M. D. Participation of 5-HT3, 5-HT4, and nicotinic receptors in the peristaltic reflex of guinea pig distal colon. Am. J. Physiol. Gastrointest. Liver Physiol.271, G849–G857 (1996). CASGoogle Scholar
- Heredia, D. J., Dickson, E. J., Bayguinov, P. O., Hennig, G. W. & Smith, T. K. Localized release of serotonin (5-Hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology136, 1328–1338 (2009). CASPubMedGoogle Scholar
- Yadav, V. K. et al. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat. Med.16, 308–312 (2010). This study showed that pharmacological inhibition of the synthesis of 5-HT from enteroendocrine cells did not reduce gastrointestinal transit in conscious mice. CASPubMedPubMed CentralGoogle Scholar
- Li, Z. et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J. Neurosci.31, 8998–9009 (2011). This study showed that mutation of the geneTph1that synthesizes mucosal 5-HT did not reduce gastrointestinal transit in conscious mice but mutation ofTph2(neuronal 5-HT) did; however, Tph2mutant mice also had developmental problems in the ENS. CASPubMedPubMed CentralGoogle Scholar
- Heredia, D. J. et al. Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J. Physiol.591, 5939–5957 (2013). CASPubMedPubMed CentralGoogle Scholar
- Vincent, A. D., Wang, X. Y., Parsons, S. P., Khan, W. I. & Huizinga, J. D. Abnormal absorptive colonic motor activity in germ free mice is rectified by butyrate, an effect possibly mediated by mucosal serotonin. Am. J. Physiol. Gastrointest. Liver Physiol.315, G896–G907 (2018). CASPubMedGoogle Scholar
- Bertrand, P. P. Real-time measurement of serotonin release and motility in guinea pig ileum. J. Physiol.577, 689–704 (2006). CASPubMedPubMed CentralGoogle Scholar
- Keating, D. J. & Spencer, N. J. Release of 5-hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology138, 659–670 (2010). CASPubMedGoogle Scholar
- Gwynne, R. M., Clarke, A. J., Furness, J. B. & Bornstein, J. C. Both exogenous 5-HT and endogenous 5-HT, released by fluoxetine, enhance distension evoked propulsion in guinea-pig ileum in vitro. Front. Neurosci.8, 301 (2014). PubMedPubMed CentralGoogle Scholar
- Tuladhar, B. R., Kaisar, M. & Naylor, R. J. Evidence for a 5-HT3 receptor involvement in the facilitation of peristalsis on mucosal application of 5-HT in the guinea pig isolated ileum. Br. J. Pharmacol.122, 1174–1178 (1997). CASPubMedPubMed CentralGoogle Scholar
- Spencer, N. J. et al. Mechanisms underlying distension-evoked peristalsis in guinea pig distal colon: is there a role for enterochromaffin cells? Am. J. Physiol. Gastrointest. Liver Physiol.301, G519–G527 (2011). CASPubMedGoogle Scholar
- Keating, D. J. & Spencer, N. J. What is the role of endogenous gut serotonin in the control of gastrointestinal motility? Pharmacol. Res.140, 50–55 (2018). PubMedGoogle Scholar
- Tsuji, S., Anglade, P., Ozaki, T., Sazi, T. & Yokoyama, S. Peristaltic movement evoked in intestinal tube devoid of mucosa and submucosa. Jpn J. Physiol.42, 363–375 (1992). CASPubMedGoogle Scholar
- Spencer, N. J., Dickson, E. J., Hennig, G. W. & Smith, T. K. Sensory elements within the circular muscle are essential for mechanotransduction of ongoing peristaltic reflex activity in guinea-pig distal colon. J. Physiol.576, 519–531 (2006). CASPubMedPubMed CentralGoogle Scholar
- Lomax, A. E. et al. Correlation of morphology, electrophysiology and chemistry of neurons in the myenteric plexus of the guinea-pig distal colon. J. Auton. Nerv. Syst.76, 45–61 (1999). CASPubMedGoogle Scholar
- Neunlist, M., Michel, K., Aube, A. C., Galmiche, J. P. & Schemann, M. Projections of excitatory and inhibitory motor neurones to the circular and longitudinal muscle of the guinea pig colon. Cell Tissue Res.305, 325–330 (2001). CASPubMedGoogle Scholar
- Bertrand, P. P. Real-time detection of serotonin release from enterochromaffin cells of the guinea-pig ileum. Neurogastroenterol. Motil.16, 511–514 (2004). CASPubMedGoogle Scholar
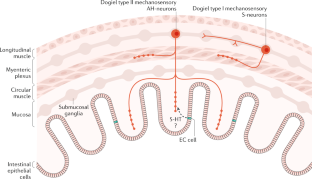
- Alcaino, C. et al. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc. Natl Acad. Sci. USA115, E7632–E7641 (2018). This study showed that EC cells are mechanosensitive and express the major ion channel Piezo 2. CASPubMedGoogle Scholar
- Baumgartner, H. R. 5-Hydroxytryptamine uptake and release in relation to aggregation of rabbit platelets. J. Physiol.201, 409–423 (1969). CASPubMedPubMed CentralGoogle Scholar
- Mawe, G. M. & Hoffman, J. M. Serotonin signalling in the gut–functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol.10, 473–486 (2013). CASPubMedPubMed CentralGoogle Scholar
- Coates, M. D., Tekin, I., Vrana, K. E. & Mawe, G. M. Review article: the many potential roles of intestinal serotonin (5-hydroxytryptamine, 5-HT) signalling in inflammatory bowel disease. Aliment. Pharmacol. Ther.46, 569–580 (2017). CASPubMedGoogle Scholar
- Raghupathi, R. et al. Identification of unique release kinetics of serotonin from guinea-pig and human enterochromaffin cells. J. Physiol.591, 5959–5975 (2013). CASPubMedPubMed CentralGoogle Scholar
- Strege, P. R. et al. Sodium channel NaV1.3 is important for enterochromaffin cell excitability and serotonin release. Sci. Rep.7, 15650 (2017). PubMedPubMed CentralGoogle Scholar
- Wang, F. et al. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J. Physiol.595, 79–91 (2017). This study showed that loss of Piezo 2 led to a loss of responsiveness to mechanical force in EC cells. CASPubMedGoogle Scholar
- Wu, J., Lewis, A. H. & Grandl, J. Touch, tension, and transduction – the function and regulation of Piezo ion channels. Trends Biochem. Sci.42, 57–71 (2017). Google Scholar
- Alcaino, C., Farrugia, G. & Beyder, A. Mechanosensitive Piezo channels in the gastrointestinal tract. Curr. Top. Membr.79, 219–244 (2017). CASPubMedPubMed CentralGoogle Scholar
- Mazzuoli-Weber, G. et al. Piezo proteins: incidence and abundance in the enteric nervous system. Is there a link with mechanosensitivity? Cell Tissue Res.375, 605–618 (2019). CASPubMedGoogle Scholar
- Kaelberer, M. M. et al. A gut-brain neural circuit for nutrient sensory transduction. Science361, eaat5236 (2018). This study suggests that enteroendocrine cells communicate to the vagal afferent nerve endings via synaptic release of glutamate. PubMedPubMed CentralGoogle Scholar
- Spencer, N. J., Smith, C. B. & Smith, T. K. Role of muscle tone in peristalsis in guinea-pig small intestine. J. Physiol.530, 295–306 (2001). CASPubMedPubMed CentralGoogle Scholar
- Spencer, N. J., Hennig, G. W. & Smith, T. K. A rhythmic motor pattern activated by circumferential stretch in guinea-pig distal colon. J. Physiol.545, 629–648 (2002). CASPubMedPubMed CentralGoogle Scholar
- Spencer, N. J., Hennig, G. W. & Smith, T. K. Stretch-activated neuronal pathways to longitudinal and circular muscle in guinea pig distal colon. Am. J. Physiol. Gastrointest. Liver Physiol.284, G231–G241 (2003). CASPubMedGoogle Scholar
- Costa, M. et al. New insights into neurogenic cyclic motor activity in the isolated guinea-pig colon. Neurogastroenterol. Motil.29, 1–13 (2017). CASPubMedGoogle Scholar
- Ellis, M., Chambers, J. D., Gwynne, R. M. & Bornstein, J. C. Serotonin and cholecystokinin mediate nutrient-induced segmentation in guinea pig small intestine. Am. J. Physiol. Gastrointest. Liver Physiol.304, G749–G761 (2013). CASPubMedGoogle Scholar
- Huizinga, J. D. et al. The origin of segmentation motor activity in the intestine. Nat. Commun.5, 3326 (2014). PubMedPubMed CentralGoogle Scholar
- Gwynne, R. M. & Bornstein, J. C. Mechanisms underlying nutrient-induced segmentation in isolated guinea pig small intestine. Am. J. Physiol. Gastrointest. Liver Physiol.292, G1162–G1172 (2007). CASPubMedGoogle Scholar
- Farthing, M. J. Enterotoxins and the enteric nervous system–a fatal attraction. Int. J. Med. Microbiol.290, 491–496 (2000). CASPubMedGoogle Scholar
- Lundgren, O. 5-Hydroxytryptamine, enterotoxins, and intestinal fluid secretion. Gastroenterology115, 1009–1012 (1998). CASPubMedGoogle Scholar
- Vanden Broeck, D., Horvath, C. & De Wolf, M. J. Vibrio cholerae: cholera toxin. Int. J. Biochem. Cell Biol.39, 1771–1775 (2007). CASPubMedGoogle Scholar
- Fung, C., Ellis, M. & Bornstein, J. C. Luminal cholera toxin alters motility in isolated guinea-pig Jejunum via a pathway independent of 5-HT3 receptors. Front. Neurosci.4, 162 (2010). CASPubMedPubMed CentralGoogle Scholar
- Koussoulas, K., Gwynne, R. M., Foong, J. P. P. & Bornstein, J. C. Cholera toxin induces sustained hyperexcitability in myenteric, but not submucosal, AH neurons in guinea pig Jejunum. Front. Physiol.8, 254 (2017). PubMedPubMed CentralGoogle Scholar
- Neunlist, M., Dobreva, G. & Schemann, M. Characteristics of mucosally projecting myenteric neurones in the guinea-pig proximal colon. J. Physiol.517, 533–546 (1999). CASPubMedPubMed CentralGoogle Scholar
- Wood, J. D. Physiology of the Gastrointestinal Tract. (ed Johnson, L. R.) Vol. 1 Ch. 21, 629–669 (Elsevier, Inc., 2012).
- Furness, J. B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol.9, 286–294 (2012). CASPubMedGoogle Scholar
- Galligan, J. J. Pharmacology of synaptic transmission in the enteric nervous system. Curr. Opin. Pharmacol.2, 623–629 (2002). CASPubMedGoogle Scholar
- Zhou, Y. & Danbolt, N. C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm.121, 799–817 (2014). CASPubMedPubMed CentralGoogle Scholar
- Wood, J. D. in Handbook of Physiology Vol. 2 Physiology of the Gastrointestinal Tract (ed. Said Hamid M.) Ch. 15 361–272 (Academic Press, 2018).
- Ren, J., Hu, H. Z., Liu, S., Xia, Y. & Wood, J. D. Glutamate receptors in the enteric nervous system: ionotropic or metabotropic? Neurogastroenterol. Motil.12, 257–264 (2000). CASPubMedGoogle Scholar
- Swaminathan, M., Hill-Yardin, E. L., Bornstein, J. C. & Foong, J. P. P. Endogenous glutamate excites myenteric calbindin neurons by activating Group I metabotropic glutamate receptors in the mouse colon. Front. Neurosci.13, 426 (2019). PubMedPubMed CentralGoogle Scholar
- Hu, H. Z. et al. Slow excitatory synaptic transmission mediated by P2Y1 receptors in the guinea-pig enteric nervous system. J. Physiol.550, 493–504 (2003). CASPubMedPubMed CentralGoogle Scholar
- Gwynne, R. M. & Bornstein, J. C. Electrical stimulation of the mucosa evokes slow EPSPs mediated by NK1 tachykinin receptors and by P2Y1 purinoceptors in different myenteric neurons. Am. J. Physiol. Gastrointest. Liver Physiol.297, G179–G186 (2009). CASPubMedPubMed CentralGoogle Scholar
- Monro, R. L., Bertrand, P. P. & Bornstein, J. C. ATP participates in three excitatory postsynaptic potentials in the submucous plexus of the guinea pig ileum. J. Physiol.556, 571–584 (2004). CASPubMedPubMed CentralGoogle Scholar
- Gwynne, R. M. & Bornstein, J. C. Synaptic transmission at functionally identified synapses in the enteric nervous system: roles for both ionotropic and metabotropic receptors. Curr. Neuropharmacol.5, 1–17 (2007). CASPubMedPubMed CentralGoogle Scholar
- Monro, R. L., Bornstein, J. C. & Bertrand, P. P. Slow excitatory post-synaptic potentials in myenteric AH neurons of the guinea-pig ileum are reduced by the 5-hydroxytryptamine7 receptor antagonist SB 269970. Neuroscience134, 975–986 (2005). CASPubMedGoogle Scholar
- Crist, J. R., He, X. D. & Goyal, R. K. Both ATP and the peptide VIP are inhibitory neurotransmitters in guinea-pig ileum circular muscle. J. Physiol.447, 119–131 (1992). CASPubMedPubMed CentralGoogle Scholar
- Mutafova-Yambolieva, V. N. et al. Beta-nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc. Natl Acad. Sci. USA104, 16359–16364 (2007). CASPubMedGoogle Scholar
- Mutafova-Yambolieva, V. N. & Sanders, K. M. Appropriate experimental approach is critical for identifying neurotransmitter substances: application to enteric purinergic neurotransmission. Am. J. Physiol. Gastrointest. Liver Physiol.309, G608–G609 (2015). CASPubMedPubMed CentralGoogle Scholar
- Wang, G. D. et al. β-Nicotinamide adenine dinucleotide acts at prejunctional adenosine A1 receptors to suppress inhibitory musculomotor neurotransmission in guinea pig colon and human jejunum. Am. J. Physiol. Gastrointest. Liver Physiol.308, G955–G963 (2015). PubMedPubMed CentralGoogle Scholar
- Wood, J. D. Response to Mutafova-Yambolieva and Sanders. Am. J. Physiol. Gastrointest. Liver Physiol.309, G610–G611 (2015). CASPubMedPubMed CentralGoogle Scholar
- Bornstein, J. C., Costa, M. & Furness, J. B. Synaptic inputs to immunohistochemically identified neurones in the submucous plexus of the guinea-pig small intestine. J. Physiol.381, 465–482 (1986). CASPubMedPubMed CentralGoogle Scholar
- Reed, D. E. & Vanner, S. J. Converging and diverging cholinergic inputs from submucosal neurons amplify activity of secretomotor neurons in guinea-pig ileal submucosa. Neuroscience107, 685–696 (2001). CASPubMedGoogle Scholar
- Foong, J. P., Parry, L. J., Gwynne, R. M. & Bornstein, J. C. 5-HT1A, SST1, and SST2 receptors mediate inhibitory postsynaptic potentials in the submucous plexus of the guinea pig ileum. Am. J. Physiol. Gastrointest. Liver Physiol.298, G384–G394 (2010). CASPubMedGoogle Scholar
- Koussoulas, K., Swaminathan, M., Fung, C., Bornstein, J. C. & Foong, J. P. P. Neurally released GABA Acts via GABAC receptors to modulate Ca 2+ transients evoked by trains of synaptic inputs, but not responses evoked by single stimuli, in myenteric neurons of mouse ileum. Front. Physiol.9, 97 (2018). PubMedPubMed CentralGoogle Scholar
- Sang, Q. & Young, H. M. The identification and chemical coding of cholinergic neurons in the small and large intestine of the mouse. Anat. Rec.251, 185–199 (1998). CASPubMedGoogle Scholar
- Tonini, M., Frigo, G., Lecchini, S., D’Angelo, L. & Crema, A. Hyoscine-resistant peristalsis in guinea-pig ileum. Eur. J. Pharmacol.71, 375–381 (1981). CASPubMedGoogle Scholar
- Tonini, M., Costa, M., Brookes, S. J. & Humphreys, C. M. Dissociation of the ascending excitatory reflex from peristalsis in the guinea-pig small intestine. Neuroscience73, 287–297 (1996). CASPubMedGoogle Scholar
- Costa, M. et al. Neurogenic and myogenic motor activity in the colon of the guinea pig, mouse, rabbit, and rat. Am. J. Physiol. Gastrointest. Liver Physiol.305, G749–G759 (2013). CASPubMedGoogle Scholar
- Costa, M. et al. Neuromechanical factors involved in the formation and propulsion of fecal pellets in the guinea-pig colon. Neurogastroenterol. Motil.27, 1466–1477 (2015). CASPubMedGoogle Scholar
- Lasrado, R. et al. Lineage-dependent spatial and functional organization of the mammalian enteric nervous system. Science356, 722–726 (2017). This study used single-cell transcriptomics and mutagenesis to provide major insights into how the ENS develops. An overlap in expression of regulatory programmes determines cell fates, where developing neurons are organized by clonal lineages. CASPubMedGoogle Scholar
- Spencer, N. J. et al. Identification of a rhythmic firing pattern in the enteric nervous system that generates rhythmic electrical activity in smooth muscle. J. Neurosci.38, 5507–5522 (2018). This study showed that the ENS generates a rhythmic firing pattern that generates rhythmic electrical activity in colonic smooth muscle that underlies propulsion of content. CASPubMedGoogle Scholar
- Li, Z. et al. Regional complexity in enteric neuron wiring reflects diversity of motility patterns in the mouse large intestine. eLife8, e42914 (2019). This study showed that there are regional differences in the intrinsic neuronal wiring patterns between the proximal and distal region of the colon. PubMedPubMed CentralGoogle Scholar
- Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci.8, 1263–1268 (2005). CASPubMedGoogle Scholar
- Deisseroth, K. Optogenetics. Nat. Methods8, 26–29 (2011). CASPubMedGoogle Scholar
- Kim, C. K., Adhikari, A. & Deisseroth, K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat. Rev. Neurosci.18, 222–235 (2017). CASPubMedPubMed CentralGoogle Scholar
- Hibberd, T. J. et al. Optogenetic induction of colonic motility in mice. Gastroenterology155, 514–528 (2018). This study demonstrated that wireless optogenetics can be used to stimulate the ENS and increase colonic transit in conscious, freely moving animals. PubMedPubMed CentralGoogle Scholar
- Boesmans, W., Hao, M. M. & Vanden Berghe, P. Optogenetic and chemogenetic techniques for neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol.15, 21–38 (2018). PubMedGoogle Scholar
- Perez-Medina, A. L. & Galligan, J. J. Optogenetic analysis of neuromuscular transmission in the colon of ChAT-ChR2-YFP BAC transgenic mice. Am. J. Physiol. Gastrointest. Liver Physiol.317, G569–G579 (2019). PubMedGoogle Scholar
- Spencer, N. J., Hibberd, T., Feng, J. & Hu, H. Optogenetic control of the enteric nervous system and gastrointestinal transit. Expert Rev. Gastroenterol. Hepatol.13, 281–284 (2019). CASPubMedPubMed CentralGoogle Scholar
- Owen, S. F., Liu, M. H. & Kreitzer, A. C. Thermal constraints on in vivo optogenetic manipulations. Nat. Neurosci.22, 1061–1065 (2019). This study showed that even very small changes in temperature (as occurs when using optogenetics) can change the behaviour of conscious animals. CASPubMedPubMed CentralGoogle Scholar
- Iyer, S. M. et al. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat. Biotechnol.32, 274–278 (2014). CASPubMedPubMed CentralGoogle Scholar
- Cannon, W. B. The movements of the stomach studied by means of the Roetgen rays. Am. J. Physiol.1, 359–382 (1898). Google Scholar
- Legros and Onimus. Recherches experimentales sur les mouvements de l’intestine. J. de l’Anat. et Physiol. 37–66 (1869).
- Langley, J. N. in Textbook of Physiology (ed. Schaffer, E. A.) 616–696 (Pentland, 1900).
Acknowledgements
H.H. was supported by grants from the NIH, R01GM101218, R01DK103901 and R01AA027065, Washington University School of Medicine Digestive Disease Research Core Center (NIDDK P30 DK052574), The Center for the Study of Itch of the Department of Anaesthesiology at Washington University School of Medicine. N.J.S. is supported by NH&MRC of Australia, grants APP1156427 and APP1156416.
Author information
Authors and Affiliations
- College of Medicine and Public Health & Centre for Neuroscience, Flinders University, Adelaide, Australia Nick J. Spencer
- Department of Anesthesiology, The Center for the Study of Itch, Washington University School of Medicine, St Louis, MO, USA Hongzhen Hu
- Nick J. Spencer









